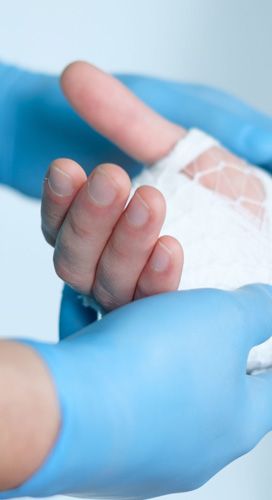
FIND OUT MORE ABOUT HUMAN TISSUE FOR HUMAN WOUNDS
Expedited wound closure with amniotic tissue between
4-6 weeks on average
AMNIOTIC KEY FEATURES & PROPERTIES:
*Our products are being used to treat wounds, burns and other complex acute and chronic wounds including diabetic ulcer, venous and pressure sores.
*Dehydrated extracellular matrix acts as a scaffolds supporting the native tissue.
*Immune privileged and angiogenic
*5-year shelf life at ambient temperature storage
*Confirmed by the FDA Tissue Reference Group to meet the criteria for regulation solely under Section 361 of PHS Act as defined in 21 CFR Part 1271.
* Amniotic Membrane allograft derived from a prescreened mother with a planned C-section delivery.
*The membrane is minimally processed to preserve the native structure of the tissue, dehydrated, and terminally sterilized.
OUR PRODUCTS
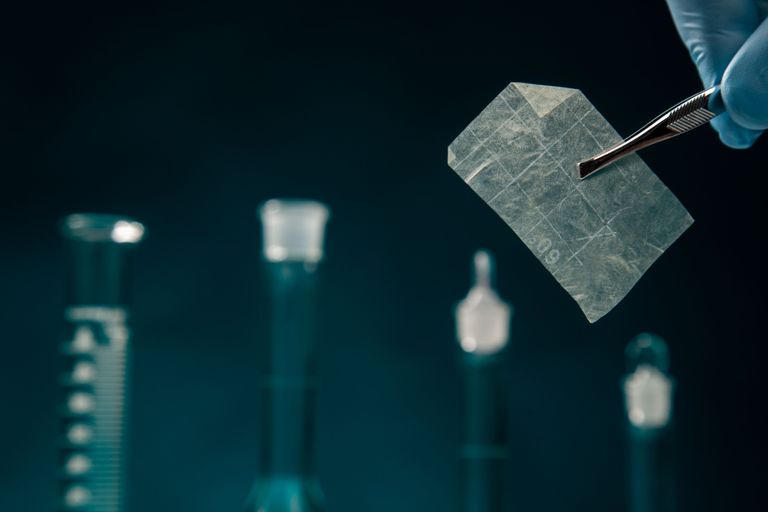
AMNIOTIC MEMBRANE
Amniotic Wound Care Matrix is a cross-linked human amniotic membrane allograft consisting of an epithelial basal and compact fibroblast layer associated with the membrane.
Amniotic Matrix provides a protective covering and a support layer for cell attachment and ingrowth during healing of diabetic ulcers, venous ulcers, pressure ulcers, burn, and other dermal ulcerations that include wounds with exposed vital structures such as tendon, muscle, or bone, with minimal adhesion and scarring.
KERECIS FISH SKIN
Kerecis Omega3 is intact fish skin rich in naturally occurring Omega3 polyunsaturated fatty acids. When grafted onto damaged human tissue such as a burn or a diabetic wound, the material recruits the body’s own cells and is ultimately converted into living tissue.
Compared to mammalian-based skin substitutes, Kerecis Omega3 offers improved economics and clinical performance, as well as reduced disease transfer risk and no cultural constraints on usage.
Other tissue-transplant products are based on tissues of human and porcine origin. These are not ideal substitutes because heavy processing is needed to eliminate the risk of disease transmission. This harsh, anti-viral treatment removes most of the material’s natural components, making it dissimilar to human skin.
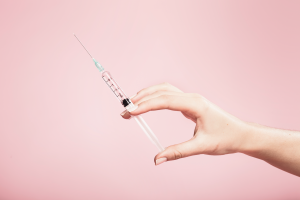
AMNIO INJECTION
Our amnio injections are an innovative treatment for foot and ankle pains, and is a non-surgical solution! Amnio injections can eliminate the need for steroids. During an amniotic fluid injection, amniotic stem cells are injected near damaged tissues to help them regenerate.
They reduce inflammation and can greatly decrease recovery time. Amniotic injections encourage tissue growth and healing of the damaged area. They are an anti-inflammatory agent and can prevent the need for surgery by stimulating the body’s natural healing mechanisms. Amniotic fluid injections signal to the amniotic stem cells in our body to flood the damaged area with oxygen and nutrients. This flow of oxygen helps new tissues grow.
Amniotic fluid injections treat the root cause of pain and regenerate our tissues on a cellular level; they also can prevent injuries from recurring! Amniotic injections can provide pain relief and encourage the cells to accelerate their healing process therefore shortening overall recovery time

BILING SERVICES
Our wide range of experience in marketing, management and financial services allows us to work in all different industries, from large to small corporations or starting businesses. We specialize in all healthcare specialties including personal injury with law firms and other medical professionals. Our company is designed to empower your team and outfit them with the tools they need to succeed. Talk to us today about how we can support your growth, to increase your per patient revenue by offering the current technology on wound healing.
We take care of the following: Insurance credentialing and contracting, claims tracking and submissions, Billing tasks and analytics, reclaiming declining revenues, claim scrubbing, Patient calls and statements, Maximus productivity, Collection services, Eligibility and benefits verification, posting of insurance and patient payments, Denial review and management, Appeals and management reports
GENERAL INFORMATION
Reimbursement and coverage eligibility for the use of the Amniotic Membrane and associated procedures varies by Medicare and private payers. Coverage policies, prior authorizations contract terms, billing edits, and site-of-service influence reimbursement.
Amniotic Membrane (Q4253) is not included on the Medicare Part B Average Sales Price (ASP) Drug pricing file published quarterly by the Center for Medicare and Medicaid Services )CMS) Average sales price information is published quarterly by the Centers for Medicare and Medicaid Services (CMC) in the ASP Medicare Part B Drug Pricing File or Not Otherwise Classified (NOC) Pricing file. Providers are encouraged to review the ASP pricing files posted quarterly by CMS and listed by HCPCS on CMS.gov for updates. Payment allowance limits that are not included in the ASP Medicare Part B Drug pricing file or not otherwise classified Pricing file, are based on the published wholesale acquisition cost or invoice pricing. In determining the payment limit based on WAC, the contractor follow the methodology specified in publication

Our Independent Contractors
© Copyright. All rights reserved.























